Ionic Liquids
Designing Liquids with New Properties – From Fundamentals to Applications
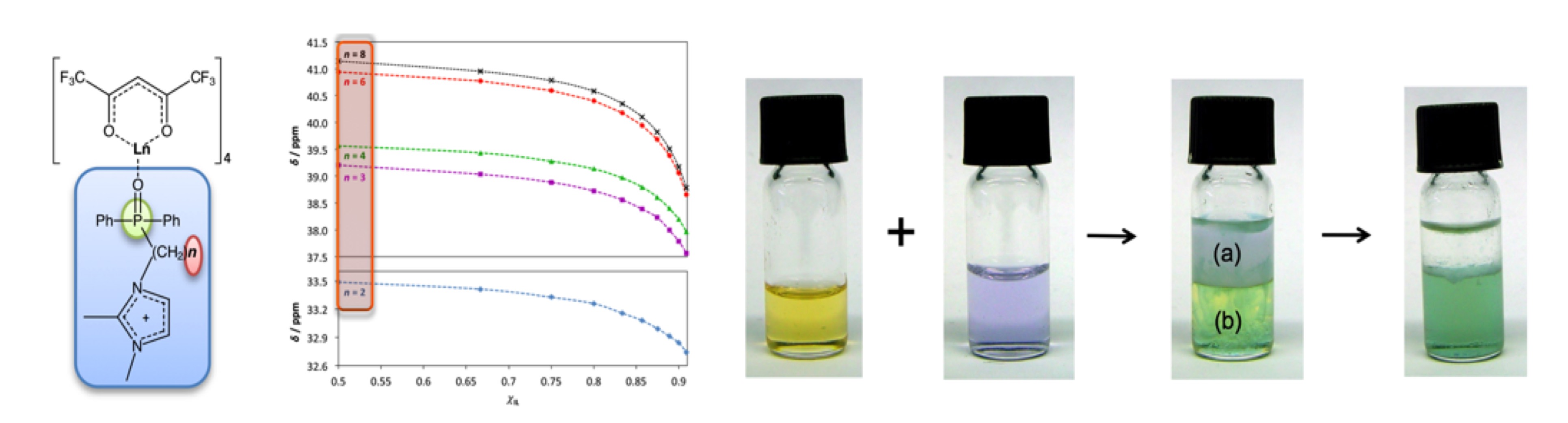
Ionic liquids can provide interesting and unique possibilities for inorganic and materials chemists. For instance, metal containing ionic liquids are regarded as promising new materials combining properties of ionic liquids and additional intrinsic magnetic, catalytic or e.g. spectroscopic properties depending on the respective included metal ion. Functionalised ionic liquids have been demonstrated as reaction media for the synthesis of inorganic compounds with unusual properties as well as for the synthesis of coordination polymers and for the crystallisation and crystal engineering of novel coordination compounds.
Figure 1: Liquid lanthanide complexes (left), a switchable cobalt-containing ionic liquid (right).
- J. Osborne; S. Wellens; C. Ward; S. Felton; R. M. Bowman; K. Binnemans; M. Swadzba-Kwasny; H. Q. N. Gunaratne; P. Nockemann: Thermochromism and switchable paramagnetism of cobalt(II) in thiocyanate ionic liquids.Dalton Trans., 2015, 44, 11286-11289.
- Nockemann; M. Pellens; K. Van Hecke; L. Van Meervelt; J. Wouters; B. Thijs; E. Vanecht; T. N. Parac-Vogt; H. Mehdi; S. Schaltin; J. Fransaer; S. Zahn; B. Kirchner; K. Binnemans: Cobalt(II) Complexes of Nitrile-Functionalized Ionic Liquids.Chemistry-a European Journal, 2010, 16, 1849-1858.
- Nockemann; B. Thijs; N. Postelmans; K. Van Hecke; L. Van Meervelt; K. Binnemans: Anionic rare-earth thiocyanate complexes as building blocks for low-melting metal-containing ionic liquids.Journal of the American Chemical Society, 2006, 128, 13658-13659.
Ionothermal Synthesis
New Pathways to Advanced Functional Materials and Nanomaterials
Ionic liquids are expanding their use to new areas such as materials chemistry and crystal engineering. Crystallisation strategies using ionic liquids are quite different from conventional organic solvents. Ionic liquids are not only replacing conventional solvents, but are also able to act as neutral solvents, templates, reactants or charge compensating species. This opens up alternative synthetic and crystallization pathways – the synthesis in low-melting salts can be seen in analogy to solid-state synthesis using a salt as a flux. However, the temperatures for ionothermal synthesis are significantly lower ranging from room temperature up to ca. 500 K, which enables new possibilities for a “gentle” solid state synthesis of inorganic compounds, for example with unusual coordination modes, low oxidation states or stabilisation of metastable compounds.
Figure 2: Microwave synthesis of nanomaterials (left), In2Se3 from ILs (middle) and a hexanuclear nickel complex with molecular magnetic properties from ionothermal synthesis (right).
One of the challenges in the synthesis of semiconductor nanocrystals suitable for solar energy conversion is the control of their composition and morphology. Ionic liquids have the ability to dissolve most semiconductor precursors, including elemental chalcogenides, can actively participate in the capping and structuring during nanoparticle growth and can withstand high temperatures without degradation.
- Tyrrell; G. Behrendt; P. Nockemann: Ionothermal Syntheses of Nano- and Microstructured Ternary Copper-Indium-Chalcogenides.Inorganic Chemistry, 2015, 54, 4495-4503.
- Tyrrell; M. Swadzba-Kwasny; P. Nockemann: Ionothermal, microwave-assisted synthesis of indium(III) selenide.Journal of Materials Chemistry A, 2014, 2, 2616-2622.
- Swadzba-Kwasny; L. Chancelier; S. Ng; H. G. Manyar; C. Hardacre; P. Nockemann: Facile in situ synthesis of nanofluids based on ionic liquids and copper oxide clusters and nanoparticles.Dalton Trans. 2012,41, 219-227.
- Nockemann; B. Thijs; K. Van Hecke; L. Van Meervelt; K. Binnemans: Polynuclear metal complexes obtained from the task-specific ionic liquid betainium bistriflimide.Crystal Growth & Design, 2008, 8, 1353-1363.
Extraction and Separation of Critical Metals
Developing More Sustainable Separation Processes for Recycling and Urban Mining
Several metals belong to the group of critical raw materials which have a high risk in the security of supply and economic importance. Rare earth metals, for example, are widespread worldwide, but rarely accumulate in concentrations high enough for economic mining; at the same time, they have widespread usage e.g. in electronics, displays, magnets in hybrid cars and windmills, superconductors, in batteries and as catalysts. Recycling rates for many critical metals are currently low and involve challenging separation processes using strong acids, harsh conditions and volatile solvents. Ionic liquids have a great potential as alternative and more environmentally benign solvents for the selective rare and precious high-tech metal extraction, separation and processing. We are exploring and evaluating the utilisation of novel functionalised ionic liquids as alternative and environmentally benign separation media, e.g. to efficiently do “urban mining” utilising electronic metal scrap as an industrial waste stream.
Figure 3: Liquid lanthanide complexes (left), a switchable cobalt-containing ionic liquid (right).
- A. Vicente; A. Mlonka; H. Q. N. Gunaratne; M. Swadzba-Kwasny; P. Nockemann: Phosphine oxide functionalised imidazolium ionic liquids as tuneable ligands for lanthanide complexation.Chemical Communications 2012, 48, 6115-6117.
- Nockemann; R. Van Deun; B. Thijs; D. Huys; E. Vanecht; K. Van Hecke; L. Van Meervelt; K. Binnemans: Uranyl Complexes of Carboxyl-Functionalized Ionic Liquids.Inorganic Chemistry, 2010, 49, 3351-3360.
- Nockemann; B. Thijs; S. Pittois; J. Thoen; C. Glorieux; K. Van Hecke; L. Van Meervelt; B. Kirchner; K. Binnemans: Task-specific ionic liquid for solubilizing metal oxides.Journal of Physical Chemistry B, 2006, 110, 20978-20992.
Energy Storage
Improving Electrolytes for Efficient Redox Flow Batteries
As the demand for and implementation of renewable energy grows, so too does demand for solutions which can store this energy in order to regulate when it is used. Redox Flow Batteries are fast becoming a preferred choice for suppliers, especially for sources of renewable energies. Vanadium Redox Flow Batteries have advantages over other systems due to their scalability, lifespan, the immediate energy release, excellent charge retention (up to 1 year), and the ability to discharge 100% with no damage. A major cost factor and limitation for the next generation of redox-flow batteries based on vanadium is the electrolyte, which is often the issue for the lower energy densities of VRBs in comparison to other battery types. Our research into new formulation of electrolytes allows us to significantly improved energy densities, that is currently one of the limitations of these redox-flow battery systems. These electrolytes have also advantages in terms of their low flammability and electrochemical long-term stability.

Figure 4: Schematic view of a vanadium redox flow battery (© P.N.).
- Belhocine; S. A. Forsyth; H. Q. N. Gunaratne; M. Nieuwenhuyzen; P. Nockemann; A. V. Puga; K. R. Seddon; G. Srinivasan; K. Whiston: 3-Methylpiperidinium ionic liquids.Physical Chemistry Chemical Physics, 2015, 17, 10398-10416.
- Leys; C. S. P. Tripathi; C. Glorieux; S. Zahn; B. Kirchner; S. Longuemart; K. C. Lethesh; P. Nockemann; W. Dehaen; K. Binnemans: Electrical conductivity and glass formation in nitrile-functionalized pyrrolidinium bis(trifluoromethylsulfonyl)imide ionic liquids: chain length and odd-even effects of the alkyl spacer between the pyrrolidinium ring and the nitrile group.Physical Chemistry Chemical Physics, 2014,16, 10548-10557.
Technology and Knowledge Transfer
Academic Entrepreneurship for ‘Green’ and Sustainable Innovation
As a director of QUB spin-out company Green Lizard Technologies Ltd. (GLT), my interest is also in taking research innovation in the clean energy and green and sustainable chemistry sector further into application and eventually commercialisation. While I used to believe that academic research and entrepreneurship would be incompatible, my involvement in several industrial projects (such as the removal of mercury from natural gas1) made me conclude that application oriented thinking can also inspire academic research and feed back into education.
Figure 6: Pilot plants for scale-up (Green Lizard Technologies Ltd).
In GLT, we explore and develop further applications, scale-up and commercialisation of metal separations, energy storage, vegetable oil refinement, waste valorisation, plastics recycling and more.
- M. Abai; M. P. Atkins; A. Hassan; J. D. Holbrey; Y. Kuah; P. Nockemann; A. A. Oliferenko; N. V. Plechkova; S. Rafeen; A. A. Rahman; R. Ramli; S. M. Shariff; K. R. Seddon; G. Srinivasan; Y. Zou: An ionic liquid process for mercury removal from natural gas. Dalton Trans. 2015, 44, 8617-8624.