The REMSIL (Rare Earth Metal Separation with Ionic Liquids) project focuses on the design and development of novel technologies based on task specific ionic liquids (TSILs), solid-supported-ionic liquids (SSILs) and ionogel membranes for the separation and recovery of high-tech rare earth metals (REMs).With the current demand for these high-tech metals and their severely limited supply in the EU, their recycling has become vital. Separation and recovery of REMs from secondary sources (“urban mining”) has become a necessity.
Ionic liquids have great potential as alternative and environmentally benign solvents for high-tech metal extraction, separation and processing. In the first stage, novel task specific ionic liquids (TSILs) with specific REM coordinating abilities will be synthesised.
Model systems for technologically important REM separations are being evaluated, to demonstrate significantly improved separations for these systems over conventional solvents.Understanding the principles governing solubility of rare earth in ionic liquids is of vital importance for extraction processes and will be studied using a combination of spectroscopic techniques. These results feed back into the design of improved TSILs. In the third stage, solid supported ionic liquids (SSILs) are developed to evaluate their potential for REM separations in industrial-scale processes.
The potential of novel TSILs with specific coordinating abilities for rare earth metal extraction and separation, including fundamental studies to improve understanding of the underpinning extraction mechanisms will be explored. The objective is to develop a new generation of cost-efficient and environmental friendly ionic liquids that pave the way to develop new rare earth metal separation technologies. Our Marie Sklodowska-Curie fellow Dr Ritesh Ruhela transfered knowledge in the design and synthesis of rare-earth metal selective ionic liquids and in metal separation technologies to the Host Institute, Queen’s University Belfast (QUB).
Rare earth metals (REMs) shape the way we live and belong to the critical metals which are technologically important and are used in everyday green energy products such as hybrid cars, wind turbines, next generation solar cells, rechargeable batteries as well as energy saving LED lighting and flat screen displays. Rapidly increasing demand has recently strained supply of REM, and there is growing concern that the world may soon face a serious shortage of the rare earths.
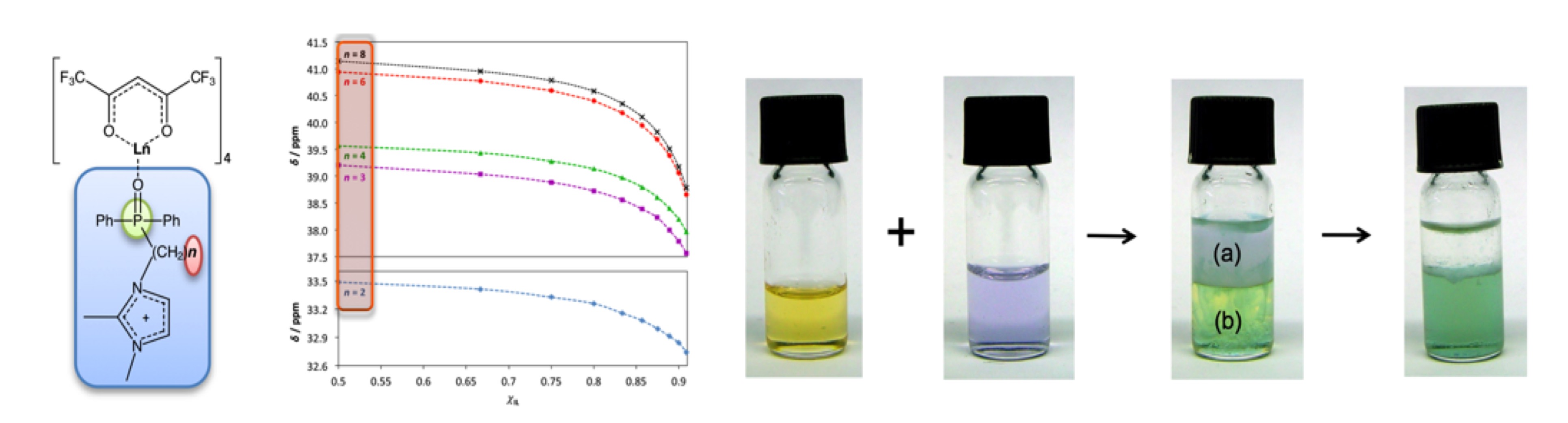
Based on our recent breakthrough at QUB on the development of novel, highly selective extractants based on new extractants for the REM separation with ionic liquids, which have more than two orders of magnitude higher selectivity, we are currently setting up a lab demonstration for a counter-current extraction process.
Ionic liquids have a great potential as alternative and more environmentally benign solvents for the selective rare and precious high-tech metal extraction, separation and processing. We are exploring and evaluating the utilisation of novel functionalised ionic liquids as alternative and environmentally benign separation media, e.g. to efficiently do “urban mining” utilising electronic metal scrap as an industrial waste stream.
Figure 1: Liquid lanthanide complexes (left), rare earth metal extraction with ionic liquids (right).
Current REM mining and recycling technology is costly and has severe environmental issues due to the use of toxic chemicals and a high number of separation stages. We aim to drastically reduce the processing costs by reducing the number of separation stages by more than one order of magnitude and uses non-toxic extractants to lower the environmental impact of the REM separation.
As a result of this programme, we hope to be able to demonstrate the feasibility of a new, cost-efficient and environmentally benign process for the rare earth metal separation, which has the potential to completely change the way these metals are processed in mining and and the extend REMs are recycled.
This project has been funded by:
Marie Skłodowska-Curie actions – Research Fellowship Programme